
This section will discuss the characteristics, advantages, and disadvantages of these promiscuous cyclases asparaginyl endoproteases, sortases, and subtilisin-like variants. These enzymes have been further engineered to cyclize a wide variety of backbone macrocyclic peptides.

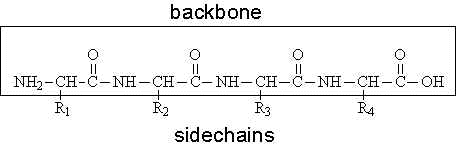
Naturally occurring backbone macrocyclic peptides are generally matured from their linear counterparts by their specific cyclases, some of which have substrate promiscuity, and may be utilized for cyclization of other peptides. In any case, this review will also cover the discussion of such technologies. Some technologies covered in this review have been well-established and successfully applied to discover bioactive molecules in the last decade, but some technologies emerged recently and have thus not yet been fully extended to the discovery of de novo macrocyclic peptides. This introduces at least one saturated single bond, expanding their flexibility compared to backbone macrocyclic peptides ( Horton et al., 2000).ĭespite the fact that there are numerous kinds of backbone macrocyclic peptides originating from naturally occurring peptides and rationally or semi-rationally designed molecules, this chapter focuses on discussing recent technical advancements that allow researchers to discover de novo backbone macrocyclic peptides. On the other hand, the other methods of cyclization use an amino acid's sidechain. Among them, a backbone macrocyclic conformation provides the most conformationally constrained structure due in part to the consecutive, unsaturated amide bonds, which cannot rotate and thus contribute to a more rigid structure. Macrocyclic peptides are classified into three groups by cyclization style: sidechain-to-sidechain, head-to-sidechain, and head-to-tail (backbone) cycles are all found in natural products. Their rigid structure contributes to the avoidance of proteolysis ( March et al., 1996), and a closed conformation, wherein hydrophobic regions are exposed to the surface but hydrophilic regions are hidden inside of the cyclic structure, increases their cell-membrane permeability ( Rezai et al., 2006). Macrocyclic peptides can potentially overcome these challenges. However, peptides consisting of ordinary amino acids have challenges to overcome before being an effective therapeutic agent, such as low metabolic stability caused by proteolysis in vivo, and poor cell-permeability caused by their larger size, and the aquaphilic property due to multiple hydrogen bonding donors/acceptors in the peptide backbone compared to small molecules. Peptides would have a specific and high binding affinity to target proteins of interest and could bind not only to their pocket but also to the relatively flat protein surface ( Laraia et al., 2015). Even though they are small in size compared with biological drugs, such as antibodies, they possess unique traits similar to those. Peptides have the potential to be therapeutic agents in various aspects. We also discuss screening methodologies compatible with those synthetic methodologies, such as one-beads one-compound (OBOC) screening compatible with the synthetic method 2, cell-based assay compatible with 3, limiting-dilution PCR and mRNA display compatible with 4.
#PEPTIDE BACKBONE CODE#
Here, we overview recent technical advancements in the synthetic methods including 1) enzymatic synthesis, 2) chemical synthesis, 3) split-intein circular ligation of peptides and proteins (SICLOPPS), and 4) in vitro translation system combined with genetic code reprogramming. Recent advances in synthetic methods for backbone macrocyclic peptides have enabled the discovery of novel peptide drug candidates against diverse targets. Therefore, such peptide scaffolds are an attractive platform for drug-discovery endeavors. Department of Chemistry, Graduate School of Science, The University of Tokyo, Tokyo, Japanīackbone macrocyclic structures are often found in diverse bioactive peptides and contribute to greater conformational rigidity, peptidase resistance, and potential membrane permeability compared to their linear counterparts.Koki Shinbara, Wenyu Liu, Renier Herman Pieter van Neer, Takayuki Katoh and Hiroaki Suga *


 0 kommentar(er)
0 kommentar(er)
